Mot Diagram Of Oxygen
Chemical bonding O2 oxygen o22 molecular mot molecule configuration electronic stabilities compare orbitals bonding bo atomic form 38 o2 2- molecular orbital diagram
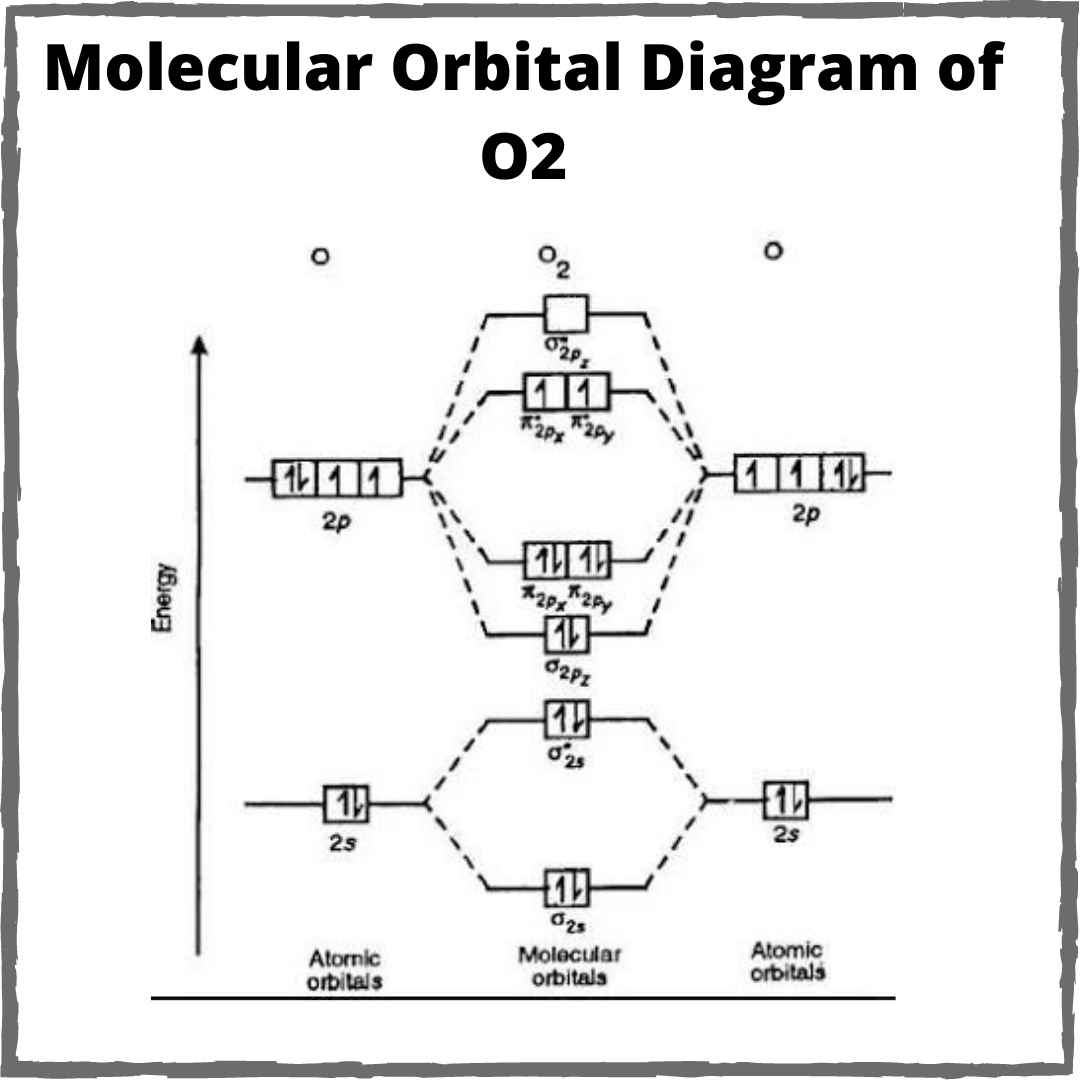
Explain the formation of ${O_2}$ molecule using molecular orbital theory.
O2 molecules there are 1 sigma bond and 1 pi bond and 2 lone pair of Out of $\\text{ }{{\\text{c}}_{2}}\\text{ }$, $\\text{ }{{\\text{f}}_{2 Orbital molecular o2 mot o22 cbse follows text electrons
O2 molecule paramagnetic calculate orbitals sarthaks
Molecular molecule o2 orbital configuration formation theoryO2 orbital order chemistry molecule crack Mot nitrogen oxygen bondingDraw the m.o diagram for oxygen molecule and calculate its bond order.
Diagram orbital molecular oxygen bonding orbitals molecule bond order sigma structure energy level bh2 anti o2 antibonding chemistry pi theoryExplain the formation of ${o_2}$ molecule using molecular orbital theory. More stable among o2+ and o2-Orbital molecular diagram mo o2 oxygen o3 radical diagrams c2 configuration electron orbitals bonding electrons draw energy dioxygen level odd.

O2 configuration electronic molecular mot n2 stable bonding bond molecule order oxygen structure o22 orbital diagram electron electrons chemistry write
Compare the stabilities of o2 , o2-,o22- .
.
38 o2 2- molecular orbital diagram - Wiring Diagram Info

Oxygen - Chemwiki

Mot

Compare the stabilities of O2 , O2-,O22- - Home Work Help - Learn CBSE

Out of $\\text{ }{{\\text{C}}_{2}}\\text{ }$, $\\text{ }{{\\text{F}}_{2

More stable among O2+ and O2- - Home Work Help - Learn CBSE Forum

Explain the formation of ${O_2}$ molecule using molecular orbital theory.
.png)
O2 molecules there are 1 sigma bond and 1 pi bond and 2 lone pair of